Monday, March 29, 2010
Sunday, March 28, 2010
Enzyme lab data and Cellular Respiration and Photosynthesis fill-in
LAB INFO
HOT:
height of bubbles: 1.5 cm
radius of test tube: 1.25 cm
Volume of reaction: 7.363 cm3
ROOM TEMP:
height of bubbles: 12 cm
radius of test tube: 1.25 cm
volume of reaction: 58.9 cm3
COLD:height of bubbles: 13 cm
radius of test tube: 1.25 cm
volume of reaction: 63.81 cm3
Effect of pH on catalase reactions
ACID (pH of 5):
height of bubbles: 12 cm
radius of test tube: 1.25 cm
volume of reaction: 58.9 cm3
NEUTRAL (pH of 7):
height of bubbles: 13.5 cm
radius of test tube: 1.25 cm
volume of reaction: 66.267 cm
BASE (pH of 9.5):
height of bubbles: 14.5 cm
radius of test tube: 1.25 cm
volume of reaction: 71.176 cm
Friday, March 26, 2010
Due March 30th
2. Word pairings # 2 is due.
3. Lab is due. Post information on blog if necessary.
4. Open book/open note test on ch. 9 & 10.
Wednesday, March 24, 2010
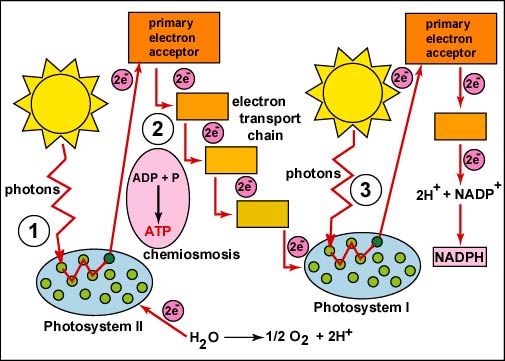
- Molecules involved in INPUT: Light, H2O
- No major enzymes
- Molecules in OUTPUT: 2 electrons, ATP
- Main objective of this stage/process
- To provide electrons for photosynthesis
- To produce ATP once it goes down the ETC
- Elaboration
- P680 molecules (described as wavelengths that represent when they absorb the most amount of light) are certain types of chlorophyll molecules that absorb energy in this process.
- When electrons that are trapped by P680 molecules get excited, they are released.
- Molecules involved in INPUT: light
- Major enzymes: NADPH (a coenzyme)
- Molecules in OUTPUT: 2 electrons, NADPH (energy-rich molecule)
- Main objective of this stage/process
- To move electrons into the Calvin Cycle to complete photosynthesis
- Elaboration
- If not enough ATP is produced, the electrons will move back into Photosystem I by the cyclic cycle
- If there is enough ATP, this starts the noncyclic cycle, which moves into the Calvin Cycle
- Molecules involved in INPUT: 6 molecules of CO2 per cycle, energy from NADPH, energy from ATP
- No major enzymes
- Molecules in OUTPUT: glucose, ADP, NADP+
- Main objectives of this stage/process
- To produce glucose
- Elaboration
- The Calvin Cycle is a light-independent reaction. This means that it does not directly need light to perform, but light must be present in order for it to perform (light comes in through phosphorylation)

Monday, March 1, 2010
PROPERTIES OF WATER
Chapter 3
Remember: Polar—unequal sharing of electrons (as in an H20 molecules—oxygen is slightly negative, hydrogen is slightly positive)
Surface tension: measure of how difficult it is to stretch or break the surface of a liquid—> hydrogen bonds

http://www.societyofrobots.com/images/robot_JB_lizard1.jpg
The so-called "Jesus Lizard"
Water can moderate temperature because of its high specific heat. Specific heat is the amount of heat required to raise or lower the temperature of a substance by 1 degree Celsius and the specific heat of water is 1 cal/g C (water has very high specific heat, so it takes more heat energy to raise the temperature of water, and more energy must be lost from water before its temperature will decrease).
It would take a huge amount of energy just to bump up the temperature of the ocean 1 degree Celsius. Water has high specific heat for this reason, because otherwise, the critters would die.
Water is also an important solvent. Review: the substance that something is dissolved in is the solvent, while the substance being dissolved is the solute. Together, they are the solution. When the solution has water as its solvent, it’s called an aqueous solution.
Substances that are water-soluble are hydrophilic substances. They are ionic compounds, polar molecules (sugars), and some proteins. Oils, however, are hydrophobic and non-polar, meaning that they do not dissolve in water.
Water is at its densest at 4 degrees Celsius.
The dissociation of water molecules
When a hydrogen atom is transferred from one water molecule to another, it leaves its electron and is transferred as a hydrogen ion, which is a proton with a charge of +1. This transfer makes the water molecule that lost its proton the hydroxide ion (OH-) and the molecule that gains the proton is the hydronium ion, H30+ (lack of it would be a base, more of it would be an acid). This dissociation of water molecules happens such that the amount of hydronium and hydroxide ions is about even in pure water. But if acids or bases are added to water, this equilibrium shifts. Water has a pH of 7, which means it’s neutral.
Buffers: substance that minimizes large sudden changes in pH.
Are combinations of H+ donors and H+ acceptor forms in solution of weak acids or bases.
Work by accepting H+ ions from solution when they are in excess and by donating H+ ions to the solution when they have been depleted.
Eg. Bicarbonate is a buffer.
Chapter 4
Carbon skeleton—Every macromolecule has a carbon skeleton.
Linear
Branch
Ring
4 macromolecules:
1. Lipids
2. Nucleic acids
3. Carbohydrates
4. Proteins
Isomers are compounds with the same molecular formula but with different structures and hence different properties. Isomers are a source of variation among organic molecules.
There are three types of isomers:
Structural, geometric, and enantiomers
Structural isomers are isomers that differ in the covalent arrangement of their atoms
Salem molecular formula, skeleton may change, and can differ in location of double bonds.
Eg. C—C=C instead of C=C—C
Geometric isomers are isomers that share the same covalent partnerships, but differ in their spatial arrangement.
Double bonds are inflexible.
Enantiomers are isomers that are mirror images of each other
Usually one form is biologically active and its mirror image is not.
FUNCTIONAL GROUPS
Hydroxyl Group
-OH and OH+ (the negative out in front is a bond sign, not a negative sign)
Carbonyl group:
Aldehyde—carbonyl is at the end off the carbon skeleton. (A for Away)
Ketone—carbonyl is in the middle or near the end of the carbon skeleton.
Carboxylic Acids:
Since this group donates protons, it has acidic properties.
Amino Group:
Characterized by nitrogen joined to atleast one alkyl group. Tends to act as a weak base.
Sulfhydrol group:
Helps stabilize the structure of protein. Disulfide bridges help stabilize tertiary structure proteins.
Organic compounds with this functional group are called thiols.
Phosphate group:
PO4 3-
Has acidic properties since it loses protons
Organic phosphates are important in cellular energy storage and transfer.